Isolation and Morphological Identification of Aspergillus Species from Some Cultivated Soils in Maiduguri, Nigeria
| Received 03 Mar, 2022 |
Accepted 30 Dec, 2023 |
Published 17 Jan, 2024 |
Background and Objective: Aspergillus is an important genus of filamentous fungus distributed worldwide with great important uses in agriculture, the environment, food industries and human health. Despite the economic importance, there is no research work reported on Aspergillus in soil from the study area. This present work was carried out to isolate and identify the various species of Aspergillus and their diversity in some cultivated soils. Materials and Methods: Representative soil samples were collected from six cultivated soils at 0-15 cm soil depth viz; vegetable crops (onion and tomato); cereal intercropped with legumes (millet/cowpea and millet/groundnut) and orchard (mango and cashew) from the Faculty of Agriculture Teaching and Research Farm and farms around the campus. Each sampled soil was analyzed for physicochemical properties and isolation of Aspergillus using the dilution plating method. The isolates were counted and identified at the species level based on morphological characteristics. Descriptive statistic was used to analyzed data and results presented in percentage. Results: Five isolates were identified; Aspergillus niger, Aspergillus flavus, Aspergillus glaucus, Aspergillus versicolor and Aspergillus ustus. Variation in species abundance amongst the sites was observed. Aspergillus niger, Aspergillus flavus and Aspergillus versicolor were common species to all the fields and thus recorded a total percent abundance of (30, 28 and 21%). Aspergillus glaucus and Aspergillus ustus had 12 and 9%, respectively and failed to appear in tomato fields. Conclusion: The results obtained indicated the presence of Aspergillus from the studied area. The highest number of Aspergillus colonies were recorded under orchards followed by cereal intercropped with legumes while vegetable crop fields recorded the least colony counts.
| Copyright © 2024 Rakiya et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Aspergillus is an important filamentous fungus distributed worldwide and can grow both on the surface of solid and liquid substrates. The fungi are commonly found in almost any environment1. Aspergillus strains form spores (conidia) under adverse conditions and are tolerant to extremely high temperature2. This spore-forming quality makes it profitable to preserve and use them as inoculants in biotechnology. Aspergillus species have been commercially used to produce organic acids, particularly citric acid3. For example, Aspergillus niger has long been used to produce citric acid for yogurts, sausages, soft drinks, wines and canned products4. While others have considerable health impacts on humans and animals. They produce gluconic acid5 which is an important chemical for the supplement of human calcium deficiencies. Some species are responsible for more than 90% of infections in humans and animals6.
The ability of some species to solubilize insoluble phosphates, such as Ca, Fe and Al phosphate was well documented by several researchers7,8. They play a huge role in the decomposition of complex plant materials and as such are generally regarded as a more sustainable means of managing organic waste. Their potential in the recycling of soil nutrients9 through, utilizing monosaccharides which creates a wide range of enzymes to hydrolyze polysaccharides, proteins, or other large organic molecules gave them important grounds in the stabilization of soil organic matter and the breakdown of residues10. The species of Aspergillus have been involved in the bioremediation of soil contaminated with heavy metals, oil spills and microbial toxins11,12. In direct applications to plants, they enhance phosphate uptake and stimulate plant growth through the production of auxins, gibberellins and other phytohormone-like compounds. In addition, many Aspergillus species have been exploited to produce a variety of important industrial enzymes, including amyloglucosidase, amylase, glucosidases, protease, cellulase, hemicellulase and xylanase13,14. More than 250 Aspergillus species were reported from different parts of the world15. Isolation and incidence of Aspergillus on some important cereal grains and environment in Nigeria have been documented16,17. However, there is no scholarly report on the occurrence and diversity of Aspergillus from the soil in this study area. The present study was therefore; conducted to isolate and identify the various species of Aspergillus and their diversity in some cultivated soils. The species were identified morphologically which consist of both macroscopic and microscopic characters.
MATERIALS AND METHODS
Description of the study area: This study was conducted in Maiduguri, Nigeria from 18th July to 30th September, 2021. Maiduguri is located in the Sahel Savannah Region of Northeast Nigeria, about 350 m above sea level at Latitude 11°05'North and Longitude 13°05'East. It occupies an area of 50,778 square km with a mean annual rainfall and temperature of about 650 mm and 32°C, respectively.
Collection of soil sample: Representative soil samples were collected from six cultivated soils viz; vegetable crops (onion and tomato); cereal intercropped with legumes (millet/cowpea and millet/groundnut) and orchard (mango and cashew) from the Faculty of Agriculture Teaching and Research Farm the University of Maiduguri and farms around the campus. Eighteen soil samples (three from each field) were collected in July, 2020, during the rainy season. The soils were sampled from 0-15 cm depth from the six different fields. The vegetable crops and cereal intercropped with legume fields were fertilized with farmyard manure and mineral fertilizer while the orchards (mango and cashew) were fertilized with only farmyard manure. Each soil sample from the six sites was shared in two; the first part was for the analysis of soil physicochemical properties and the second part was sent to the Microbiology Laboratory University of Maiduguri for isolating pure cultures of Aspergillus.
Analysis of soil physicochemical properties: The soil was air dried and sieved through a 2 mm sieve used for the determination of the physicochemical properties of the soil. The texture of the soil was determined by the Bouyoucos hydrometer method18, pH by using 1:2.5 soil-water extract and determined with a pH meter, electrical conductivity (EC) was determined on the extract for the pH using a conductivity meter, organic carbon by wet oxidation method, Walkley and Black19, total nitrogen by micro Kjeldahl procedure, as described by Horneck and Miller20 available phosphorus by Bray and Kurtz method21, exchangeable bases determined after extraction with ammonium acetate. Calcium (Ca) and Magnesium (Mg) were determined by EDTA titration method22, while potassium (K) was by flame photometer23 at the Soil Science Laboratory, University of Maiduguri.
Isolation and morphological identification of Aspergillus isolates: Using the dilution plating method24, 1 g representative sample of soil, was diluted in 9 mL sterile water and 1 mL of this was seeded onto each figure of Potato Dextrose Agar (PDA). Plates were incubated at 25°C. After the first 48 hrs, figures were examined daily for 7 days. All fungi considered to represent Aspergillus species were then subcultured onto freshly prepared Potato Dextrose Agar (PDA) plates for phenotypic identification. Isolates were identified through macroscopic observations (color and nature of the fungal growth both on the surface of the growth medium and on the reverse side) and microscopic observations (nature of hyphae, presence or absence of spores and other microscopic structures), with the aid of a light microscope using 10 and 40× objective lens and published guidelines25,26. The total number of colonies from each site was counted and recorded. By summing up all the individual abundance of Aspergillus recorded from a site the total CFU g–1 was obtained. Shannon-Wiener’s index (H¢) was used to measure the species diversity in all the sites as described by Keylock27.
Statistical analysis: Descriptive statistic was used to interpret data using measures of frequencies and results presented in percent.
RESULTS
Soil physicochemical: The physical and chemical characteristics of the studied sites were presented in Table 1. Soil pH ranges from 6.71-7.23 which is neutral with low EC indicating no salinity problem. The soils had low % OC which ranges from 0.41-1.21%. The N, P, K and CEC were higher in cereal intercropping farming fields and orchards.
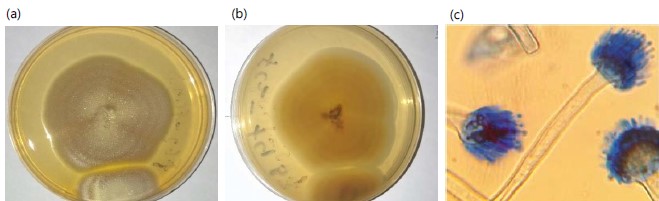
Morphological identification: Based on the morphological characteristics five isolates were identified. Aspergillus versicolor are obverse and reverse views on potato dextrose agar, which is variously colored and may range from very pale green, pinkish green, greenish beige, and salmon green. The reverse is reddish to uncolored and the growth rate is slow Fig. 1(a-b). The microscopically hyphae are septate and hyaline. Conidial heads are biseriate and loosely radiate. Conidiophores are hyaline to pale brown smooth-walled walled and brittle. vesicles are small and variably shaped (Fig. 1c).
Aspergillus glaucus are obverse and reverse views of colonies on potato dextrose agar that are greyish- turquoise to deep green with yellow central areas, the reverse is pale yellow to pale brown Fig. 2(a-b).
| Table 1: | Physical and chemical characteristics of soil from experimental sites at 0-15 cm depth | |||
Soil sample collection sites |
||||||
| Soil characteristics Soil type |
Onion Sandy loam |
Tomato Loamy |
Millet/Cowpea Loamy |
Millet/Groundnut Loamy sand |
Mango Loamy |
Cashew Loamy sand |
| pH (1:2.5) | 6.71 |
6.82 |
7.23 |
7.03 |
7.1 |
7.01 |
| EC (dS m–1) | 0.46 |
0.49 |
0.61 |
0.61 |
0.59 |
0.53 |
| OC (%) | 0.41 |
0.45 |
0.52 |
0.67 |
1.21 |
1.1 |
| N (g kg–1) | 0.51 |
0.54 |
1.22 |
1.03 |
0.82 |
0.71 |
| P (mg kg–1) | 0.91 |
0.96 |
1.03 |
1.11 |
1.21 |
1.1 |
| K (cmol kg–1) | 0.19 |
0.18 |
0.2 |
0.2 |
0.19 |
0.21 |
| Ca (cmol kg–1) | 4.61 |
4.52 |
4.71 |
4.2 |
4.4 |
4.2 |
| Mg (cmol kg–1) | 4.21 |
4.3 |
4.52 |
4.11 |
4.22 |
3.91 |
| CEC (cmol+kg–1) | 10.13 |
11.22 |
12.57 |
13.01 |
13.63 |
13.32 |
| EC: Electrical conductivity, OC: Organic carbon, N: Nitrogen, P: Phosphorus, K: Potassium, Ca: Calcium, Mg: Magnesium and CEC: Cation exchange capacity | ||||||
|

|
|
Microscopically hyphae are septate and hyaline. Conidial heads radiate to loosely columnar. Conidiophores are smooth-walled, uncolored to pale brown. Vesicles are uniseriate, globose to sub-globose and phialides cover the upper portion of the vesicle (Fig. 2c).
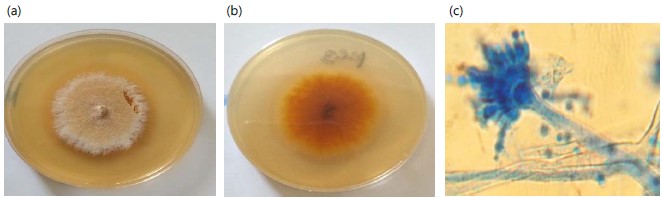
Aspergillus ustus are obverse and reverse views of potato dextrose agar colonies are white to yellow to drab grey or brown. The reverse is yellow to brown. The texture is cottony to granular Fig. 3(a-b). Microscopically hyphae are septate and hyaline. Conidial heads radiate to loosely columnar and biseriate. Conidiophores are smooth-walled and brown, and vesicles are globose to globose sub-globose. Metule and phialides cover the upper portion of the vesicle. Conidia are globose and rough-walled (Fig. 3c).
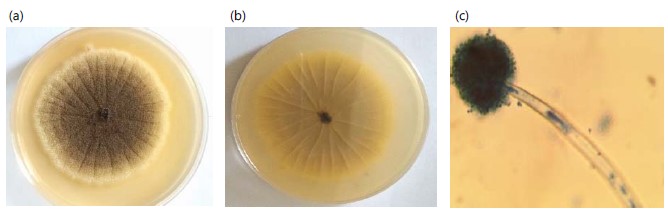
Aspergillus niger are obverse and reverse view, colonies on potato dextrose agar attained a diameter of 4-5 cm within 7 days. It consists of a compact white or yellow basal felt with a dense layer of dark brown to black conidiophores Fig. 4(a-b). Conidiophore stipes are smooth-walled, hyaline, and brown. Phialides are borne on metulae. Conidia globose and rough-walled (Fig. 4c).
Aspergillus flavus are obverse and reverse view, colonies on potato dextrose agar attained a diameter of 3-5 cm within 7 days. It consists of a dense felt of yellow-green conidiophores Fig. 5(a-b). Microscopically conidiophores are hyaline and coarse, vesicles are globose. Phialides are borne directly on the vesicle. Conidia are globose to sub globose pale green and echinulate (Fig. 5c).
|

|
|
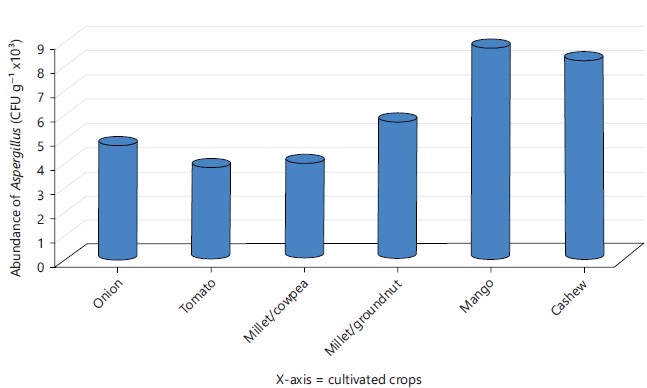
Aspergillus fungal diversity: The abundance levels of Aspergillus fungi (CFU g–1) isolated from all the sites were shown in Fig. 6. The total numbers of colonies were higher in mango and cashew fields followed by millet/groundnut fields. Tomato recorded the lowest value followed by millet/cowpea. There was variation in specie abundance amongst the sites. Aspergillus niger, Aspergillus flavus and Aspergillus versicolor were common specie in all the fields and thus recorded a total percent abundance of (30, 28 and 21%). Aspergillus glaucus and Aspergillus ustus had 12 and 9%, respectively and failed to appear in the tomato field.
DISCUSSION
Fungi are essential constituents of the soil microbiota and play a key role in soil ecosystem functioning, especially in forest and agricultural soils. The role of Aspergillus fungi in the cycling of major nutrients especially, their potential role in the decomposition of complex organic molecules and solubilization of phosphate (Ingle and Padole)28 cannot be overlooked. The results of this study revealed the presence of Aspergillus species from all the cultivated sites. However, their composition and diversity were shown to have been affected by different land use and fertilizer application. It is worth noting that the occurrence of Aspergillus in almost every environment has been reported1. They can adapt and strive to several unfavorable conditions29.
The abundance of Aspergillus species and improved soil chemical properties recorded in orchards (mango and cashew) compared to other land use as observed in this study could be attributed to the application of farmyard manure and the quantity of leaf litter turnover from the standing trees which might have increased the organic matter accumulation in the orchards. This was similarly reported by some researchers30,31. Fernan et al.32 also reported that leaf litter accumulation in the understory of mango had potential nutrients and organic carbon turnover to the soil as observed in this study. Decomposition of leaves promote the addition of soil organic matter as well as food availability for the growth of soil fungi. It is notable that agricultural soils with many fungal communities are known to have quantitative and qualitative soil organic matter improvement by Holík et al.33 and Six et al.34. Aspergillus niger and Aspergillus flavus, that were common in all the fields as observed in this study were similarly reported by Peronne et al.35. They reported Aspergillus nigri A. flavus, A. parasiticus, A. ochraceus, A. carbonarius and A. alliaceus as common in several crops. Previous studies and research conducted in Nigeria16 and Kenya36 also reported similar species as most predominant in maize and soil. The morphological method is the cheapest and most reliably assay to identify Aspergillus since other techniques are expensive. In the present study five important Aspergillus species were identified through macroscopic features of colonies and microscopic characteristics from some selected cultivated soils which is the first study of its kind in Maiduguri. Therefore, this research recommends further studies from other locations and molecular work to complement the findings of the morphological characteristics to identify many species.
CONCLUSION
The results obtained indicated the presence of Aspergillus from the studied area. The highest number of Aspergillus colonies was recorded under orchards followed by cereal-intercropped fields. Five isolates were identified; Aspergillus niger, Aspergillus flavus, Aspergillus glaucus, Aspergillus versicolor and Aspergillus ustus. Common species in all the fields were Aspergillus niger, Aspergillus flavus and Aspergillus versicolor. However, Aspergillus glaucus and Aspergillus ustus were not present in tomato fields.
SIGNIFICANCE STATEMENT
Determining the composition of microbiota and their biodiversity in soils of different types and land use is important. The genus Aspergillus includes many species and strains with several beneficial effects for healthy agricultural soils and other scientific importance. This study has identified the presence of Aspergillus species in different agricultural land use which is the first report from the study area, to our knowledge. The research has also contributed to a better understanding of how the application of organic manure imparts a positive influence on the species biodiversity of Aspergillus and improved soil nutrient composition. It is recommended that a molecular study should be conducted to identify several species and strains with the potential for use as bio-fertilizers and bioremediation of polluted soils.
REFERENCES
- Samson, R.A., C.M. Visagie, J. Houbraken, S. ,B Hong and V. Hubka et al., 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol., 78: 141-173.
- Wyatt, T.T., M.R. van Leeuwen, H.A.B. Wösten and J. Dijksterhuis, 2014. Mannitol is essential for the development of stress-resistant ascospores in Neosartorya fischeri (Aspergillus fischeri). Fungal Genet. Biol., 64: 11-24.
- Behera, B.C., R. Mishra and S. Mohapatra, 2021. Microbial citric acid: Production, properties, application, and future perspectives. Food Front., 2: 62-76.
- Show, P.L., K.O. Oladele, Q.Y. Siew, F.A.A. Zakry, J.C.W. Lan and T.C. Ling, 2015. Overview of citric acid production from Aspergillus niger. Front. Life Sci., 8: 271-283.
- Singh, O.V. and R. Kumar, 2007. Biotechnological production of gluconic acid: Future implications. Appl. Microbiol. Biotechnol., 75: 713-722.
- Segal, B.H., 2009. Aspergillosis. N. Engl. J. Med., 360: 1870-1884.
- Oliveira, C.A., V.M.C. Alves, I.E. Marriel, E.A. Gomes and M.R. Scotti, 2009. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol. Biochem., 41: 1782-1787.
- Rinu, K., M.K. Malviya, P. Sati, S.C. Tiwari and A. Pandey, 2013. Response of cold-tolerant Aspergillus spp. to solubilization of Fe and Al phosphate in presence of different nutritional sources. ISRN Soil Sci., 2013.
- Žifčáková, L., T. Větrovský, A. Howe and P. Baldrian, 2016. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol., 18: 288-301.
- Treseder, K.K. and J.T. Lennon, 2015. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev., 79: 243-262.
- Baldrian, P., 2003. Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol., 32: 78-91.
- Barros Jr., L.M., G.R. Macedo, M.M.L. Duarte, E.P. Silva and A.K.C.L. Lobato, 2003. Biosorption of cadmium using the fungus Aspergillus niger. Braz. J. Chem. Eng., 20: 229-239.
- Botella, C., A. Diaz, I. de Ory, C. Webb and A. Blandino, 2007. Xylanase and pectinase production by Aspergillus awamori on grape pomace in solid state fermentation. Process Biochem., 42: 98-101.
- Sánchez, C., 2009. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv., 27: 185-194.
- Tsang, C.C., J.Y.M. Tang, S.K.P. Lau and P.C.Y. Woo, 2018. Taxonomy and evolution of Aspergillus, Penicillium and Talaromyces in the omics era-past, present and future. Comput. Struct. Biotechnol. J., 16: 197-210.
- Campbell, C.A., I.I. Osaigbovo and R.O. Oladele, 2021. Triazole susceptibility of Aspergillus species: Environmental survey in Lagos, Nigeria and review of the rest of Africa. Ther. Adv. Infect., 8.
- Yusuf, H.O., J. Olu, A.J. Alu and T.S. Anjorin 2021. Isolation and morphologically identification of Aspergillus flavus incidences from maize seeds in Abuja, Nigeria. Fungal Territory, 4: 5-8.
- Bouyoucos, G.J., 1962. Hydrometer method improved for making particle size analyses of soils. Agron. J., 54: 464-465.
- Walkley, A. and I.A. Black, 1934. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci., 37: 29-38.
- Horneck, D.A. and R.O. Miller, 1997. Determination of Total Nitrogen in Plant Tissue. In: Handbook and Reference Methods for Plant Analysis, Kalra, Y.P. (Eds.), CRC Press, Boca Raton, Florida, ISBN: 9781420049398, pp: 75-83.
- Bray, R.H. and L.T. Kurtz, 1945. Determination of total organic and available forms of phosphorus in soils. Soil Sci., 59: 39-46.
- Tucker, B.B. and L.T. Kurtz, 1961. Calcium and magnesium determinations by EDTA titrations. Soil Sci. Soc. Am. J., 25: 27-29.
- Junsomboon, J. and J. Jakmunee, 2011. Determination of potassium, sodium and total alkalies in portland cement, fly ash, admixtures and water of concrete by a simple flow injection flame photometric system. J. Autom. Methods Manage. Chem.,2011.
- Pitt, J.I. and A.D. Hocking, 2009. Primary Keys and Miscellaneous Fungi. In: Fungi and Food Spoilage, Pitt, J.I. and A.D. Hocking (Eds.), Springer, New York, ISBN: 978-0-387-92207-2, pp: 53-143.
- Klich, M., 2002. Identification of Common Aspergillus Species. ASM Press, Washington, DC., ISBN: 9789070351465, pp: 116.
- Varga, J., J.C. Frisvad, S. Kocsubé, B. Brankovics, B. Tóth, G. Szigeti and R.A. Samson, 2011. New and revisited species in Aspergillus section Nigri. Stud. Mycol., 69: 1-17.
- Keylock, C.J., 2005. Simpson diversity and the Shannon-Wiener index as special cases of a generalized entropy. Oikos, 109: 203-207.
- Ingle, K.P. and D.A. Padole, 2017. Phosphate solubilizing microbes: An overview. Int. J. Curr. Microbiol. Appl. Sci., 6: 844-852.
- Sun, J.M., W. Irzykowski, M. Jedryczka and F.X. Han, 2005. Analysis of the genetic structure of Sclerotinia sclerotiorum (Lib.) de bary populations from different regions and host plants by random amplified polymorphic dna markers. J. Integr. Plant Biol., 47: 385-395.
- Allison, S.D. and J.B.H. Martiny, 2008. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. U.S.A., 105: 11512-11519.
- Swer, H., M.S. Dkhar and H. Kayang, 2011. Fungal population and diversity in organically amended agricultural soils of Meghalaya, India. J. Org. Syst., 6: 3-12.
- Fiegalan, F.T., C.L. Ringor and T.B. Moya, 2017. Collembola inoculation in soil incorporated mango leaf litter enhances decomposition for organic matter building and nutrient banking. Int. J. Sci. Res., 6: 1869-1877.
- Holík, L., L. Hlisnikovský, R. Honzík, J. Trögl, H. Burdová and J. Popelka, 2019. Soil microbial communities and enzyme activities after long-term application of inorganic and organic fertilizers at different depths of the soil profile. Sustainability,11.
- Six, J., S.D. Frey, R.K. Thiet and K.M. Batten, 2006. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J., 70: 555-569.
- Perrone, G., A. Susca, G. Cozzi, K. Ehrlich and J. Vargas et al., 2007. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol., 59: 53-66.
- Nyongesa, B.W., S. Okoth and V. Ayugi, 2015. Identification key for Aspergillus species isolated from maize and soil of Nandi County, Kenya. Adv. Microbiol., 5: 205-229.
How to Cite this paper?
APA-7 Style
Rakiya,
A., Kellu,
H.A., Oyekemi,
A.O., Benisheikh,
A.A. (2024). Isolation and Morphological Identification of Aspergillus Species from Some Cultivated Soils in Maiduguri, Nigeria. Research Journal of Microbiology, 19(1), 1-8. https://doi.org/10.3923/rjm.2024.1.8
ACS Style
Rakiya,
A.; Kellu,
H.A.; Oyekemi,
A.O.; Benisheikh,
A.A. Isolation and Morphological Identification of Aspergillus Species from Some Cultivated Soils in Maiduguri, Nigeria. Res. J. Microbiol 2024, 19, 1-8. https://doi.org/10.3923/rjm.2024.1.8
AMA Style
Rakiya
A, Kellu
HA, Oyekemi
AO, Benisheikh
AA. Isolation and Morphological Identification of Aspergillus Species from Some Cultivated Soils in Maiduguri, Nigeria. Research Journal of Microbiology. 2024; 19(1): 1-8. https://doi.org/10.3923/rjm.2024.1.8
Chicago/Turabian Style
Rakiya, Abdullahi, Haruna Ali Kellu, Akinmusire Olubamise Oyekemi, and Ali Abba Gana Benisheikh.
2024. "Isolation and Morphological Identification of Aspergillus Species from Some Cultivated Soils in Maiduguri, Nigeria" Research Journal of Microbiology 19, no. 1: 1-8. https://doi.org/10.3923/rjm.2024.1.8

This work is licensed under a Creative Commons Attribution 4.0 International License.



